
What Are the Differences Between Urea and Ammonium Sulfate? (Applicable Comparison)
higher-quality fruits and crops. Without enough nitrogen in the soil, plants can show signs like yellow leaves, dropped foliage, stunted growth, and poor yields. That’s why farmers and gardeners often use nitrogen fertilizers to boost soil levels when needed. Two popular options are urea and ammonium sulfate. They’re both effective, but they work differently based on soil type, weather, and crop needs.
In this article, we’ll break down what each fertilizer is, how they compare, and tips for using them. Urea, ammonium sulfate, and ammonium nitrate are mineral nitrogen fertilizers that are quickly absorbed by plants and can be used in a wide range of soils. Earlier, we compared ammonium sulfate and ammonium nitrate. In this article, we are going to compare ammonium sulfate and urea.
What Is Urea?
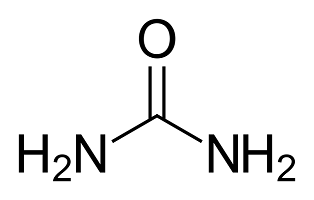
Urea is a common nitrogen fertilizer with the chemical formula CO(NH₂)₂. It has a high nitrogen content, about 46%, making it one of the most concentrated options available. This means you need less product to deliver the same amount of nitrogen compared to other fertilizers.
Urea is converted into a plant-absorbable form in three steps. In the first step, the enzymes in the soil convert urea into ammonia. After that, the formed ammonia reacts with the water in the soil, and ammonium is formed. In the third stage, ammonium is converted into nitrate through the activity of soil microorganisms. When the humidity and temperature are right, the conversion of urea to ammonium takes between 2 and 4 days. The colder the weather, the slower this process happens.
One big downside of urea is potential waste. If applied on the surface without mixing it into the soil, it can lose nitrogen as ammonia gas, especially in warm, dry, or high-pH (alkaline) conditions. Losses can be 20% or more if not managed right. Once it turns to nitrate, it can also leach away with heavy rain or irrigation since nitrate doesn’t bind well to soil particles.
To minimize losses, incorporate urea into the soil right away, or apply it just before rain or irrigation. It’s often cheaper than other nitrogen sources, but you might need to apply it in smaller doses over time to avoid waste. Urea works well for crops needing a quick nitrogen boost, like corn or grasses, but it’s not ideal for surface application on pastures in summer.
What Is Ammonium Sulfate?
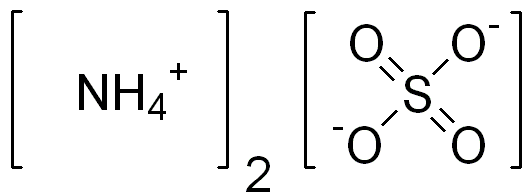
Ammonium sulfate, with the formula NH4 (2SO4), is one of the most widely used nitrogen fertilizers. Its composition consists of 21% nitrogen and 24% sulfur.
This fertilizer dissolves well in water, but its nitrogen is in the ammonium form, which binds to soil particles. That means less leaching compared to urea. It releases nitrogen more slowly and steadily, providing a reliable source over time. Ammonium sulfate also acidifies the soil more than most nitrogen fertilizers, which makes it great for alkaline or calcareous soils where lowering pH can improve nutrient availability.
Volatilization losses are low with ammonium sulfate, even when surface-applied, except on very high-pH soils (above 7.5). It’s versatile and can be mixed with other fertilizers. Use it for crops like wheat, pastures, or trees in regions where sulfur is needed, such as tropical areas for palms. As we mentioned, sulfur in ammonium sulfate reduces soil pH.
Comparison of Urea and Ammonium Sulfate
Choosing between urea and ammonium sulfate depends on your soil pH, crop type, weather, and what nutrients you need. Urea is fast-acting and cheap but needs careful handling to avoid losses. Ammonium sulfate is more stable, adds sulfur, and works better in alkaline soils, but it has lower nitrogen content, so you apply more product.
| Aspect | Urea | Ammonium Sulfate |
|---|---|---|
| Chemical Formula | CO(NH₂)₂ | (NH₄)₂SO₄ |
| Nitrogen Content | 46% | 21% |
| Sulfur Content | 0% | 24% |
| Release Type | Quick-release (converts to ammonium/nitrate in 2-4 days) | Slow-release (steady ammonium supply) |
| Leaching Risk | High (nitrate form leaches easily) | Low (ammonium binds to soil) |
| Volatilization Risk | High if surface-applied (20%+ losses possible) | Low (minimal on most soils) |
| Soil pH Effect | Mildly acidifying overall; temporary pH increase during breakdown | Strongly acidifying; good for alkaline soils |
| Best Soil Types | Acidic to neutral; avoid high pH without incorporation | Alkaline, calcareous, or sulfur-deficient |
| Cost | Cheaper per pound of nitrogen | More expensive per pound of nitrogen (but adds sulfur value) |
| Best Uses | Quick boost for corn, small grains; fall/spring applications with incorporation | Topdressing pastures/wheat; alkaline soils; tropical trees like palms |
| Disadvantages | High waste if not managed; no sulfur | Lower N concentration; more product needed; over-acidifies if overused |
- The chemical formula of urea is CO(NH2)2 and the chemical formula of ammonium sulfate is (NH4)2SO4 .
- The percentage of nitrogen in urea fertilizer is 46%. If ammonium sulfate has 21% nitrogen.
- Ammonium sulfate contains 24% sulfur, which greatly affects plant growth and health. Urea fertilizer does not contain sulfur.
- Urea is in the category of quick-release fertilizers. If ammonium sulfate is slow-release. The effect of urea is much faster than that of ammonium sulfate; therefore, this fertilizer is suitable for plants that need to receive nitrogen quickly. Of course, for fertilizing with urea, the soil must have favorable conditions. Ammonium sulfate is one of the slow-release fertilizers; over time, it has provided the plant with its nitrogen requirement. Ammonium sulfate fertilizer is suitable for supplying nitrogen to plants during the season.
- Washing and sublimation of urea is high. But ammonium sulfate has little leaching.
- Ammonium sulfate is suitable for use in alkaline and saline soils, but urea fertilizer is not. Urea fertilizer increases the alkalinity and salinity of saline and alkaline soil, making it more suitable for crops that grow in acidic soil.
- Urea fertilizer is cheaper than ammonium sulfate.
- Saline soil leads to an imbalance of nutrients, including sulfur deficiency. Ammonium sulfate contains sulfur and can supply the sulfur needed by the plant.
- Urea has a very high solubility, Which makes it easy for plants to absorb this fertilizer.
- Ammonium sulfate is more suitable for fertilizing trees in tropical regions. Its positive effects on palm trees include the growth of roots and leaves, increasing the health of the trunk, and improving the quality of the product.
In general, ammonium sulfate is a versatile choice for soils needing pH adjustment or sulfur, while urea is better for fast nitrogen in acidic soils or when cost is key. Sometimes, it is better to fertilize agricultural land using a combination of ammonium sulfate and urea. The simultaneous use of these two fertilizers not only reduces the harm of using them separately, but also increases the efficiency of nitrogen absorption.
How to Use Urea and Ammonium Sulfate

Nitrogen fertilizers such as ammonium sulfate, urea, ammonium nitrate, etc., are applied according to the plant’s needs. Always start with a soil test from a lab to check nitrogen levels, pH, and other nutrients. This helps avoid over-fertilizing, which can harm plants, cause excessive weed growth, stop root development, or pollute groundwater with nitrates.
Without a test, experienced farmers might use trial-and-error, but that’s risky. Typically, nitrogen fertilizers are applied 2-3 times a year: late winter, early spring, and during flowering or fruiting.
- For urea: Incorporate it into the soil or irrigate right after to prevent losses. Split applications to match plant needs.
- For ammonium sulfate: It can be surface-applied more safely. Monitor soil pH over time, as it acidifies, you might need lime eventually.
Remember, more isn’t better. Overuse wastes money and hurts the environment. Follow local guidelines or consult an extension service for specific rates.

