Investigating the production and manufacturing process of ammonium sulphate
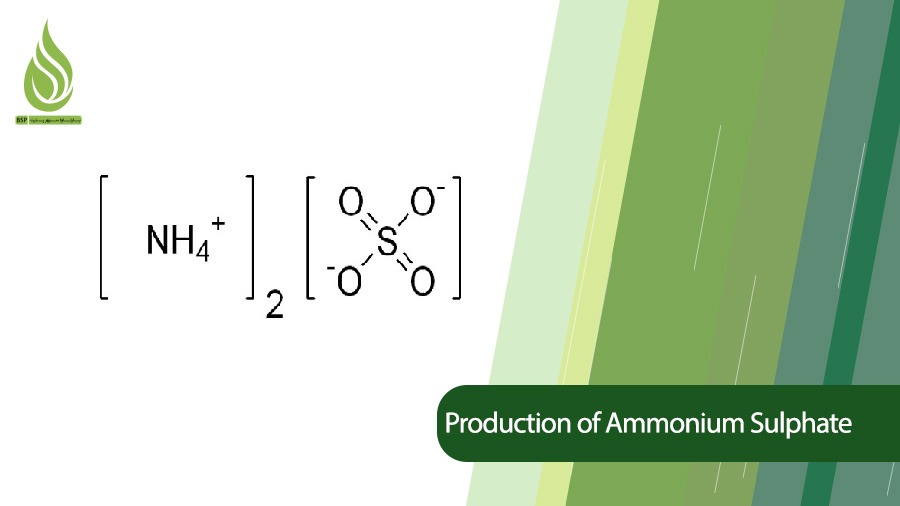
Ammonium sulphate, with the chemical formula (NH4)2SO4, is a mineral salt with many commercial applications. This material contains 21% Nitrogen and 24% Sulfur. Several hundred tons of ammonium sulphate are produced annually and are used in agriculture and other industries. In this article, we review the process of producing and manufacturing ammonium sulfate in brief.
What is ammonium sulphate?
Ammonium sulphate or sulfate is a chemical widely used in agriculture and other industries. The most important industry uses this substance. Fertilizers containing ammonium sulphate are among garden and agricultural crops’ most productive and widely used nitrogen fertilisers.
In addition to the agricultural industry, ammonium sulfate is used in other industries, such as tanning, water treatment, and chemical industries, such as the manufacture of paints, adhesives, and burglar alarms.
Ammonium sulphate’s most important and main application is strengthening alkaline soils. It keeps the amount of nitrogen in the soil at a suitable level and also increases the soil’s acidity. The color of this material varies from white to beige.
However, this product is usually sold as white crystals. The particle size of this material depends on the intended application.
Ammonium sulfate has three different grades. The grade of the product determines its application.
- Industrial grade: This product has more impurities and is used in industries such as construction, leather, wood, etc.
- Special grade: This grade is the most used because its purity is higher. It is used in agricultural applications.
- Certified grade: This product is used in the food industry, such as the pharmaceutical, livestock, and poultry industries.

Steps to make ammonium sulfate
There are two general methods for producing ammonium sulfate:
- A process where ammonium sulphate production is the main goal. (synthetic method)
- A process in which ammonium sulphate production is not the main goal, and this material is obtained as a side product.
1- Production of ammonium sulfate by synthetic method
In the synthetic method, ammonium sulphate is obtained from the ammonia and concentrated sulfuric acid reaction. The most important materials and tools in the laboratory production of ammonium sulfate include ammonia, sulfuric acid, a round-bottom flask, base, clip, magnet, heater stirrer, glass beaker, pipette, and pourer.
In the synthetic method, ammonium sulfate is produced through the reaction between sulfuric acid and heated ammonia.
Controlling the production conditions of ammonium sulfate determines the size of the grains of this material. We need a chemical process between ammonia gas (NH3) and sulphur dioxide (SO2) to produce ammonium sulfate. This process is carried out on an industrial scale.
The steps for making ammonium sulfate are as follows:
- Preparation of ammonia: Ammonia is one of the primary ingredients of ammonium sulphate. Ammonia is usually produced from natural gas, petroleum refining, or air nitrogen separation processes.
- Preparation of sulphur dioxide: SO2 is obtained from fossil fuels or chemical processes.
- Making ammonium sulphate: To make ammonium sulphate, ammonia and sulphur dioxide react chemically under appropriate temperature and pressure. This reaction is called Haber-Bosch and is NH3 + SO2 → (NH4)2SO4. In this reaction, ammonia gas turns into an ammonium ion (NH4+) and reacts with sulphur dioxide gas to a sulphate ion (SO4^2-).
- Crystal formation: After the chemical reaction, the final solution is produced as a solution. The obtained solution should go to the crystallization and crystal formation stage. The size of ammonium sulphate grains is determined by controlling the production conditions. In the production process, when the desired particle size is achieved, the ammonium sulfate grains are dried and sieved for sizing. Ammonium sulphate is produced in different forms, such as powder, crystal (fine and coarse) and granules. White spherical granule morphology is the most popular grade of this material.
- Quality control: The obtained granular ammonium sulfate grains are analysed to measure their quality. Quality control is critical at all stages.
- Packaging, storage and marketing: The final product is marketed in different packages. Bags containing ammonium sulphate should be stored in cool and dry warehouses. The storage place in the warehouse should be well-ventilated. Ammonium sulphate is available in packages of one kilogram to several kilograms.
Another method of making ammonium sulfate is to produce this product from gypsum. In this process, gypsum and ammonium carbonate are mixed, and calcium carbonate is precipitated. In this method, ammonium sulfate remains in the solution phase and is then converted into crystal ammonium sulfate in the crystallizer equipment.

2- Indirect production of ammonium sulfate
Part of the production of ammonium sulphate is the result of processes in which the production of ammonium sulfate is not the main goal, and this material is obtained as a side product. Among these processes, we can mention the production of caprolactam and methyl methacrylate.
Part of the production of ammonium sulfate is the result of processes in which the production of ammonium sulfate is not the main goal, and this material is obtained as a side product.
Ammonium sulfate manufacturing statistics in Iran and the world
The annual production capacity of ammonium sulfate in the world is about 40 million tons. The largest exporters of ammonium sulfate in the world are China, Belgium, the Netherlands, Canada and the United States. China is meeting about 15 million tons of the world’s ammonium sulphate needs. The rest of the production is done in other countries.
As we explained, ammonium sulphate is produced in two synthetic and lateral ways. Ammonium sulphate is an impurity byproduct with a lower price in the world market. Also, the price of buying and selling synthetic ammonium sulfate depends on the purity percentage of the product.
Considering currency fluctuations and the cost of importing goods, importing ammonium sulphate is not very economical. Ammonium sulfate production is a complex industrial process that requires precise equipment and processes to produce a quality final product.
Several reputable companies in Iran produce this product by relying on the latest industrial production methods. In Iran, ammonium sulphate is produced both synthetically and as a by-product. Barthava Sepehr Company, a producer of high-quality ammonium sulfate, is ready to provide you with services.
Important safety tips for employees of ammonium sulfate production units
- Avoid contact with skin and eyes.
- Rubber gloves and protective glasses must be used during production. If you do not have glasses, you must use a face shield.
- If this chemical comes into contact with the skin, wash the contact area with cold water for 15-30 minutes. Then, cover the irritated skin with an antibacterial conditioner.
- When making ammonium sulfate or working with this substance, wear a filter mask, gloves, and work clothes.
- Inhalation of this substance can cause drowsiness, shortness of breath, convulsions or muscle contraction. In case of inhaling this substance, take the person to the soldier’s environment, and if the symptoms worsen, take him to the emergency room immediately.
Conclusion
The manufacturing process of ammonium sulfate includes the combination of ammonia and sulfuric acid, crystallization, purification, and drying. This compound, which has nitrogen and sulfur, is used in agriculture as a chemical fertilizer and in various industries as a raw material, purifier, food additive, and stabilizer. Ammonium sulfate is very important in improving soil quality, enhancing plant growth, producing chemicals and food, and improving industrial processes. The manufacturing process of ammonium sulfate affects the quality and grade of this product. You should visit reputable companies like Barsava Sepehr to buy high-quality ammonium sulfate.
